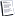
Actually the correct formula for Ideal Gas Law is PV=NRT and it is derived from Boyle's Law. P=Pressure, V=Volume, N=the Total Mass of the Gas, T=Temperature in degrees Kelvin, and R=the Ideal Gas Constant.
Now, if you use the Ideal Gas Law factors such as the Rate of Expansion for a gas can be factored in as can the Change in Volume during an event. While the math involved goes beyond simple Algebra into Differential Equations it can be used to predict the pressure at a particular instant in Time, which is exactly how programs such as Quickload work. For example give a real whiz at Differential Equations the Rate of Expansion for a particular powder (a more accurate description of what we commonly call Burn Rate), the diameter of the bullet and it's mass, and a specific instant of time after ignition and he could sit down with a pencil, paper, and simple slide rule and give you a fairly close estimate of the pressure.
So, I wouldn't say that Boyle's Law doesn't apply because it is actually at the core of a complete description of Internal Ballistics. However it is too basic for this task and in order to get to the correct solution you have to use the Ideal Gas Law and Applied Mathematics to get there.

|